The creation of new materials is one of the most important scientific thoughts and one of the main areas of work for SUSU researchers. The combination of carbon, which everyone knows, into new, never before seen forms, is what Dmiriy Zherebtsov, Candidate of Chemical Sciences, Senior Researcher of the Department of Materials Science and Physical Chemistry of Materials of the Faculty of Materials Science and Metallurgical Technologies, engineer of the Nanotechnologies Research and Education Center at SUSU, is working on.
The goal and its history
The main goal the researchers have set for themselves is the creation of a new class of carbon materials. The goal is ambitious, but the effect from achieving it will also be significant. Thirty years ago, the appearance of fullerons and nano tubes strongly pushed forward the development of physics, chemistry, and electronics. Recent discoveries turned researchers’ attention to a new class of objects – graphene and its derivatives. All of these substances are forms of carbon.
Crystal forms of carbon include graphite, diamond, and fullerons. In addition, according to theoretical calculations, the existence of a huge new class of bonds, consisting only of carbon atoms but having absolutely different forms of crystal grids, is possible.
Key characteristics and possibilities
Just like nanotubes and graphene, the new materials will be marked by unique physical features: high electrical conductivity, and high mechanical durability, a large specific surface area, significant volume of adsorption of organic and non-organic molecules, and low density.
Such materials could be in demand in, for example, in electronics, batteries, gas and solar elements, capacitors. With the help of these materials, catalysis and selective adsorption in organic synthesis, and cleaning water and air will be possible. The high specific surface area, electric capacity, and chemical inertness of the new carbon materials will make it possible to create more effective electrodes for liquid and gas sensors. It will be possible to apply them as a component of solar batteries for solar decomposition using sunlight on hydrogen and oxygen, which will make it possible for humanity to gain an ecologically-clean, renewable, non-exhaustible source of energy. The efficiency and service of the new generation of solar elements are not high yet, and silicic solar panels are less expensive, but moving them over to carbon-based panels is a matter of time. Panels from this promising carbon bonds are being created now. It is no secret that the traditional energy products (gas, coal, and especially oil) are exhaustible, and obtaining them is becoming more difficult and expensive. The cost of gas will rise. In addition, when burning carbon, we pollute the atmosphere. So, sooner or later, alternative sources of energy, including solar batteries, will become more economically profitable.
And in everyday use and production, effective adsorbents for cleaning liquids and gasses, including water and air, will come in handy.
.png)
.png)
Image 1. Image 1 shows examples of molecules with a high carbon content, which are the raw materials for obtaining new carbon materials.
SUSU is on the front lines of science!
Despite the fact that the chemical formulas of these bonds have been known for more than half a century, it was only first possible for SUSU researchers to grow the monocrystals this year. The main method for turning such molecules into new carbon materials that the researchers chose was synthesizing covalent organic frameworks (COF) from these molecules. Synthesis of COF has been rapidly developing around the world the last ten years. Up until now, a few hundred COF have been created (figure 2), but not one of them has a fully carbon-based molecular frame.
.png)
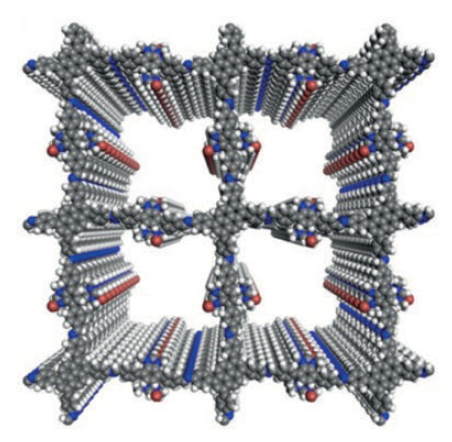
Image 2. Examples of famous COF with a layered structure
The bridges between large aromatic molecules are made from nitrogen- or oxygen-containing groups. Under heat of more than six hundred degrees Celsius, COF degrades with the destruction of these molecules and the loss of the grid-like spatial structure, turning in to flat, amorphous carbon, without any scientific or technical use.
Dmitriy Zherebtsov and his colleagues set a goal of doing something which no one has ever done before: obtain COF in which the bridge bonds between aromatic nuclei are only carbon-carbon and do not contain oxygen- or nitrogen-containing groups which do not stand up to heat. If this difficult task is completed, it will become possible to obtain carbon without damaging the oxygen skeleton of the initial material after cleavage. Even under heat up to a thousand degrees Celsius, this material will only consist of carbon atoms, arranged in space the same as they were arranged in the COF. And this is a new class of bonds which is more expansive and interesting than the classes of fullerenes and nanotubes.
It will be possible to separate layered carbon grids into separate sheets and obtain very thin membranes or filters able to let molecules of air and water through, but stop dangerous micro organisms. This can be helpful, for example, in cleaning liquids and gases, and also in medicine. For example, if this membrane was to be used as a bandage for open wounds, then air and water would pass through, while dangerous microorganisms could not, which means that the burn or wound will not get infected, and will “breathe”.
At this time, the researchers are actively studying the characteristics of related carbon materials: graphites with a large nitrogen content. If a portion of the oxygen atoms in graphite were to be replaced with nitrogen, graphite would gain new characteristics: high electrical conductivity, specific adsorption of cations of metals and organic molecules, and higher resistance to oxidation in electrochemical objects (such as batteries, capacitors, and gas and solar elements).
In their work, the SUSU researchers use special autoclaves created by their own blueprints.
Who is working on this project
Synthesis of new bonds is being worked on at the Department of Materials Science and the Physical Chemistry of Materials, and study of the characteristics is underway at the Nanotechnology Research and Education Center. Invaluable help was offered by excellent specialists in the faculties of physics, chemistry, and more at SUSU – doctors of science Vyacheslav Avdin, Yekaterina Bartashevich, Vyacheslav Yeremyashev; candidates of science Fedor Podgornov, Denis Vinnik, Kseniya Smolyakova, Sergey Morozov, and Vladimir Zhivulin, as well as Sergey Merzlov, Roman Morozov, and Dmitriy Zhivulin. Students from the Faculty of Materials Science and Metallurgical Technologies and the Faculty of Chemistry are participating in the research.
The work has attracted the attention of foreign colleagues from France, Finland, and Taiwan. Soon, highly qualified specialist Sakthi Dharan will come from India to work with the South Ural region researchers.
Researchers abroad are studying the characteristics of the graphites with high nitrogen content obtained at SUSU on special equipment. The Taiwanese and Finnish group is completing x-ray fluorescence and magnetic research of the obtained graphite. The French group is studying its electrochemical characteristics. In Moscow they are studying the structure of organic substances.
The research being held is the basis for publications in highly rated scientific journals and will become the object of applications for grants. Dmitriy Zherebtsov notes that topics for researchers will appear rom reading a large number of scientific articles, predominately in English. At the same time, an idea of new areas of science will form, and the important, unsolved tasks which have not gained the attention they deserve in the world, and which can be worked on using the equipment at SUSU without large financial costs. Synthesis of nitrogen-containing graphites in 2016-2017 cost only 200 rubles for the chemical agents.
“Read! Think! Look for your topic!” said Dmitriy Anatolyevich to his students and postgraduates, “Find a difficult task for yourself that you can solve with basic methods!”.




